
Alkanes are organic compounds that consist of single-bonded carbon and hydrogen atoms. The formula for Alkanes is CnH2n+2, subdivided into three groups – chain alkanes, cycloalkanes, and the branched alkanes.

The simple alkane methane contains one carbon atom and CH4 as its molecular formula. As this compound have just single covalent bonds only, therefore, its structural formula is

In a long chain alkane molecule, additional carbon atoms are attached to each other with the help of a single covalent bond. Each atom is attached to the sufficient hydrogen atoms to develop a total of four single covalent bonds. This long-chain structure is known as octane. An eight-carbon alkane has a molecular formula – C 8H 18 and structural formula-

The list of some Alkanes with the molecular formula and structures are given below.
Structural formulas for alkanes can be written in condensed form. For example, the structural formula of pentane contains three CH2 methylene groups in the middle of the chain. We can group them together and write the structural formula. The first five alkanes formulas with an unbranched chain are tabulated below.
Name
Molecular formula of alkane
Condensed structural formula of alkane
An abbreviated way to draw structural formulas in which each vertex and line terminus represents a carbon atom and each line represents a bond.
Formulas of organic compounds present information at several levels of sophistication. Molecular formulas, such as that of octane give the number of each kind of atom in a molecule of a compound. The molecular formula of C8H18 may apply to several alkanes, each one of which has unique chemical, physical and toxicological properties. These different compounds are designated by structural formulas showing the order in which the atoms in a molecule are arranged. Compounds that have the same molecular, but different structural formulas are called structural isomers.
Most organic compounds can be derived from alkanes. In addition, many important parts of organic molecules contain one or more alkane groups, minus a hydrogen atom, bonded as substituents onto the basic organic molecule. As a consequence of these factors, the names of many organic compounds are based on alkanes.
As with other organic compounds, the carbon atoms in alkanes may form straight chains, branched chains, or rings. These three kinds of alkanes are straight chain alkanes, branched chain alkanes and cycloalkanes. The general molecular formula of alkane for straight and branched-chain alkanes is CnH2n+2 and that of cyclic alkanes is CnH2n.
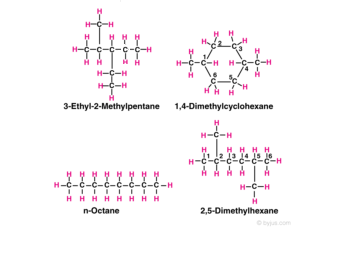
For example in the diagram, the four hydrocarbon molecules contain 8 carbon atoms each. In one of the molecules, all the carbon atoms are in a straight chain and in two they are in branched chains, whereas in a fourth, 6 of the carbon atoms are in a ring.
When a substituent like halogen bonds to an alkane molecule, one carbon-hydrogen bond of the molecule gets converted to a carbon-substituent bond. It can be understood with an example- A new compound known as chloromethane is formed when methane reacts with chlorine. The new compound is composed of a CH3 group that is bonded to a chlorine atom.
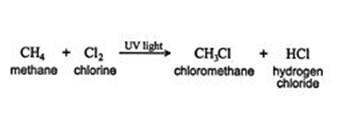
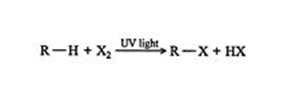
When an alkane having hydrogen is removed from one bond, it is called an alkyl group. This Alkyl group is often denoted by the letter R the same as halogens represent by the letter X. Here is a methane‐chlorine reaction that can be generalized as
Methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10) are the first four alkanes. Methane gas, whose molecular formula is CH4, is the simplest alkane.
Alkanes are single-bond hydrocarbon atoms. Three types of alkanes are available: linear straight alkanes branched alkanes and cyclic alkanes.
Usually, alkanes are not considered functional groups; rather, an alkane is a compound that lacks functional groups. A carbon-carbon double bond is a functional group in an alkene.
In a homologous series, alkynes are compounds with the general molecular formula of CnH2n‐2.
Alkynes are triple-bond carbon-carbon hydrocarbons. They do not display any geometric or optical isomerism. Ethyne (HC≡CH), frequently known as acetylene, is the easiest alkyne as shown on the right.
Learn more about hydrocarbons and their types by downloading BYJU’S – The Learning App.
Test your knowledge on alkanes!